Covering the spectrum of

iVIZIA lubricant eye drops offer an advanced formulation and innovative bottle design in an over-the-counter (OTC) solution to provide relief for your patients who suffer from dry eyes.
Your patients will see the difference with preservative-free iVIZIA that delivers a comprehensive combination of lasting relief and ocular surface protection.1-2

Chronic Dry Eye Patient Usage Study*:
Up to
8hrs
of relief
as well as improved comfort during computer work, reading, and driving3
84%
of chronic dry eye sufferers reported iVIZIA worked better than their previous eye drops3
Advantages of iVIZIA Lubricant Eye Drops

*In a chronic dry eye patient usage study, participants from a variety of socioeconomic backgrounds. answered questions about their satisfaction with iVIZIA lubricant drops. 203 chronic dry eye patients, 28-80 years old, switched from their dry eye artificial tears to iVIZIA for this month-long study.
†To limit blurriness when using contact lenses, remove contacts, apply drops, then insert contacts.
‡Source: Rx market data, Dec. 2022 - S01K without cyclosporine.
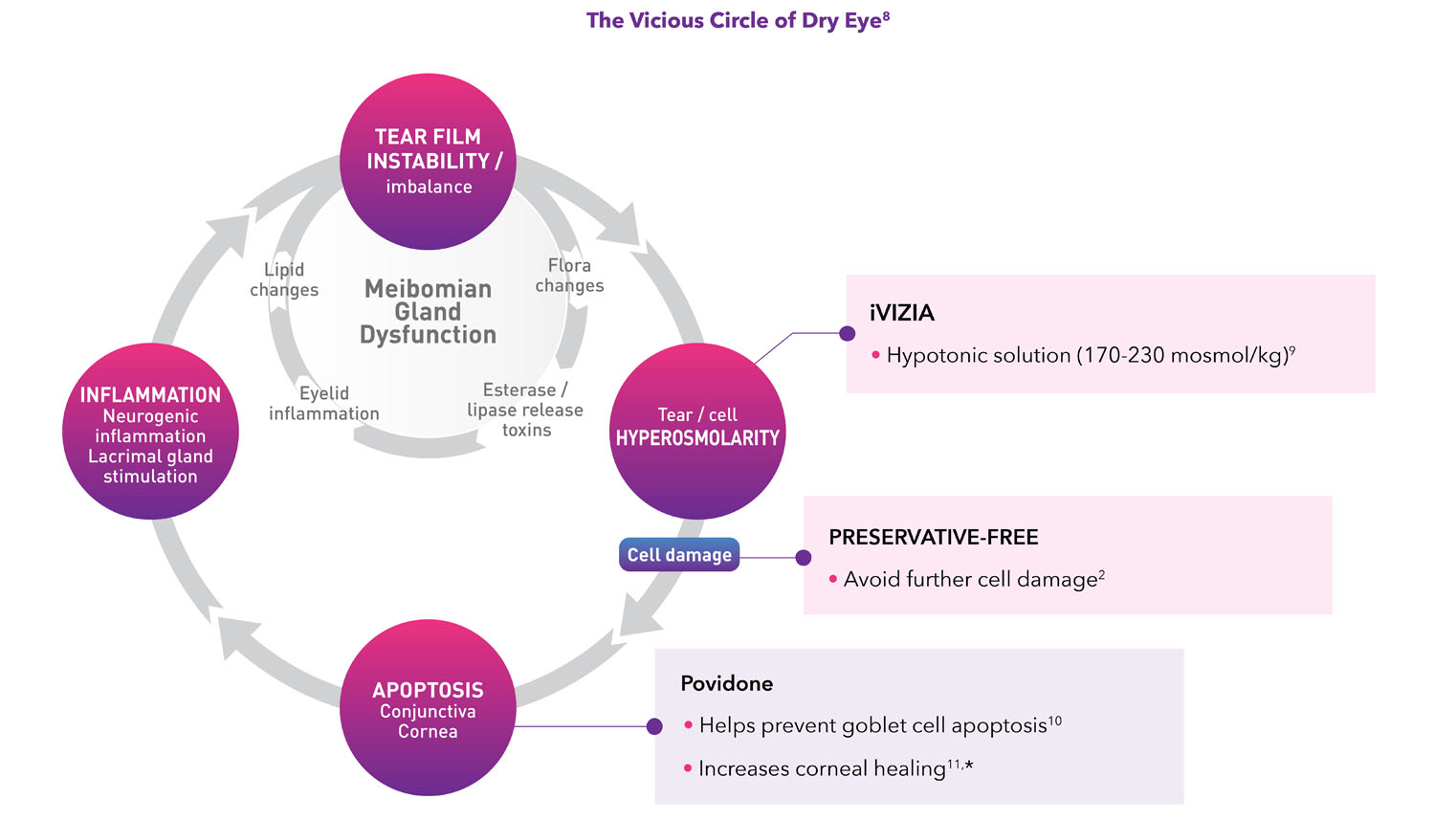
Tackle the vicious circle of dry eye

The ingredients and hypotonic solution of iVIZIA may target the vicious dry eye circle:
- Osmoregulation—allows the balance of the osmotic pressure on both sides of the cell membrane and thus limits the movement of water4
- Hypotonic formulation designed to combat hyperosmolarity in the tear film
- Prevents water leakage from cytoplasm (osmoprotection)
- Stabilizes lipid bilayers4
- Protects proteins by preventing degradation5
Help your patients see eye care differently


iVIZIA Lubricant Eye Drops—a preservative-free formulation with the active ingredient povidone
- Active ingredient enhanced by trehalose and hyaluronic acid (HA)
- Trehalose provides bioprotection, osmoprotection, and rehydration4
- HA and povidone deliver lubrication with long-lasting relief 1,6
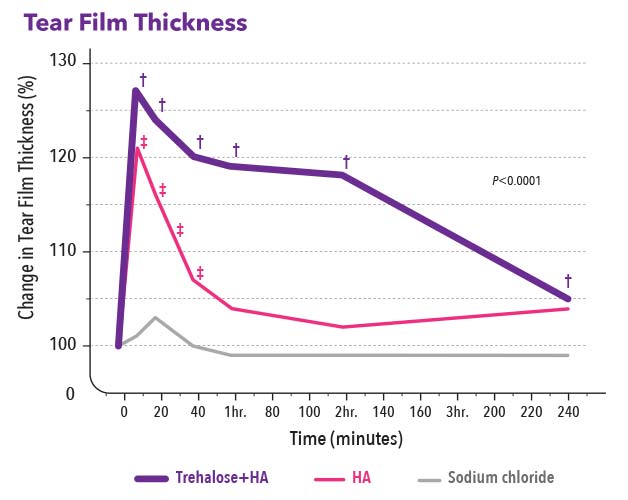
- Increased tear film thickness for up to 240 minutes7
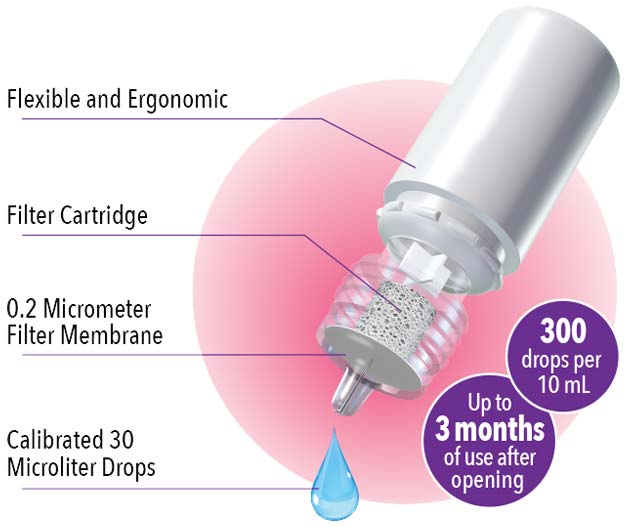
- Packaged in the proprietary ABAK® bottle—a multi-dose bottle capable of delivering calibrated eye drops
- Suitable for all dry eye sufferers, including contact lens wearers*

Eyelid Hygiene Products
iVIZIA Eyelid Cleansing Wipes—convenient daily cleansing for sensitive eyelids
- No preservatives, parabens, soaps, or perfumes
- Micellar formula gently breaks up and lifts lid oils and makeup
- Ingredients include botanicals and zinc
- No rinsing necessary after use
- Clinically shown to reduce eyelid discomfort over time12
iVIZIA Micellar Eyelid Cleanser—economical daily cleansing for eyelids
- Liquid formulation with no BAK preservatives, parabens, soaps, or perfumes
- Suitable for daily hygiene of eyelids, particularly at the base of the eyelashes
- Micellar formula gently breaks up and lifts lid oils and makeup
- No rinsing necessary after use
The iVIZIA Story

* Source: Rx market data, Dec. 2022 - S01K without cyclosporine

Open your patients’ eyes to innovative eye care with iVIZIA
References:
1. US FDA Department of Health and Human Services. Ophthalmic drug products for over-thecounter human use. Updated June 7, 2023. Accessed September 9, 2023
2. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
3. Data on file, Laboratoires Théa
4. Elbein AD, et al. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003; 13(4): 17R-27R.
5. Jain NK and Roy I. Effect of trehalose on protein structure. Protein Sci. 2009; 18:24-36
6. Pisárčik M, Bakoš D, Čeppan M. Non-Newtonian properties of hyaluronic acid aqueous solution. Colloids Surf A Physicochem Eng Asp. 1995; 97:197-202
7. Schmidl D, Schmetterer L, Witkowska KJ, et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea. 2015; 34:421-6
8. Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016; 100:300- 306
9. Data on file. Laboratoires Théa
10. Ehrenberg M, Zolotariov E, Loeb E, Poliansky V, Levy A. Combining Sodium Hyaluronate and Polyvinylpyrrolidone Therapies for the Rabbit Cornea: A New Approach to Relief of the Human Dry Eye Syndrome. Curr Eye Res. 2015; 40:913-922
11. Otto. S, Roth HW. Effectiveness and Compatibility of a polyvidone preparation treatment of wetting disorders. Klin Monbl Augenheikd 1996; 209:362-267
12. Guillon M, Maissa C, Wong S. Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Contact Lens. 2012; 38:306-312
13. Data on file. Laboratoires Théa